锂硫电池具有高的能量密度,但正极活性物质硫的低导电性和充放电中间产物多硫化物的穿梭效应以及电极的体积效应等使电池造成严重的容量衰减和库伦效率的下降,阻碍了锂硫电池高比能的发挥和商业化应用进程。
最近,我们在之前工作的基础上(ACS Applied Energy Materials, 2019, 2, 705-714.)通过静电自组装的方法制备一种中空核壳结构的MNR@HPC修饰MXene组装的三维多级结构复合材料(MCT),并作为硫正极宿主材料提高了硫正极的导电性和结构稳定性以及硫利用率;多级结构的物理限域协同化学吸附的双重作用有效缓解了多硫化物的穿梭,使电极表现出优异的倍率性能和循环稳定性。
该文章发表在国际期刊Nanoscale上,博士研究生张恒和张培根副教授为论文共同第一作者,潘龙副研究员和孙正明教授为文章的通讯作者。
文章全文链接:https://doi.org/10.1039/D0NR06151D (点击跳转到文章页)
核心内容如下:
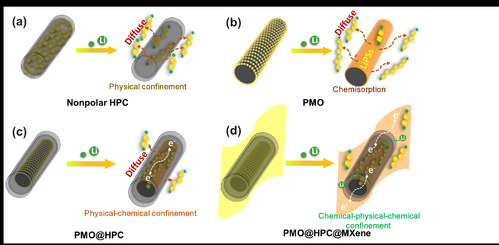 |
| 图1. 不同硫载体对多硫化物穿梭抑制示意图:(a) 中空多孔碳, (b) 极性金属氧化物, (c) 多孔碳包覆金属氧化物及(d) MXene基多层级结构 |
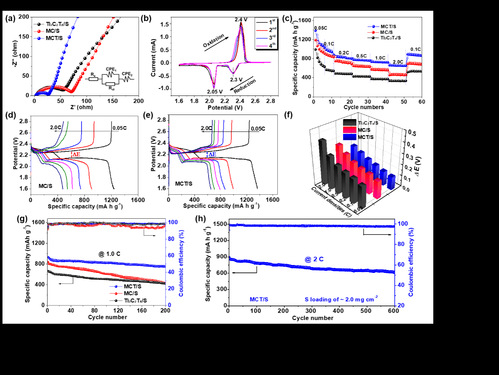 |
| 图2. 复合硫正极表现出的电化学性能:(a) 交流阻抗谱, (b) 循环伏安曲线, (c-f) 倍率性能, (g) 恒流充放电曲线和(h) 长循环性能 |
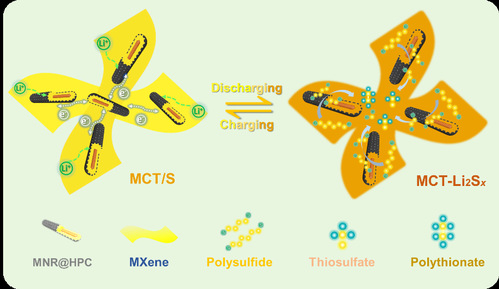 |
| 图3. MCT对充放电过程中多硫化物的吸附和转化机制示意图 |
Although the lithium-sulfur (Li–S) batteries have high energy-density, the insulative nature of elemental S, the shuttling of intermediate lithium polysulfides (LiPSs) and volume expansion of the S cathodes, resulting in capacity fading and Coulombic efficiency decrease, hinder their practical application.
Recently, we reported a multifunctional polysulfide mediator (MCT), fabricated by self-assembling 1D core–shell MnO2 nanorods@hollow porous carbon (MNR@HPC) and 2D MXene based on our previous work (ACS Applied Energy Materials, 2019, 2, 705-714.). The hierarchical structural 3D MCT, served as S host, enhanced the conductivity, structure stability and S utilization of the S cathode. The MCT/S cathode delivered an excellent rate capability and cyclic stability owing to the synergistic physical confinement and chemisorption of MCT for mitigating polysulfide shutting.
More details you can refer to: https://doi.org/10.1039/D0NR06151D.
Brief introduction:
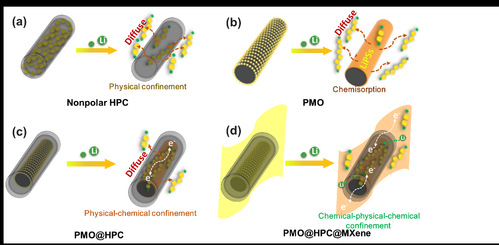 |
| Figure 1 Schematic illustration for LiPS confinement in (a) nonpolar HPC, (b) PMO, (c) PMO@HPC, and (d) PMO@HPC@MXene |
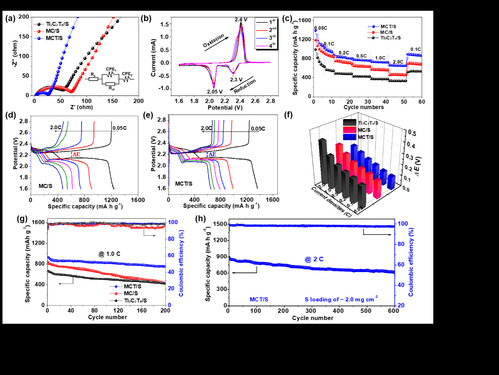 |
| Figure 2 Electrochemical performance of S cathodes: (a) Nyquist plots, (b) CV profiles, (c-f) rate capability, (g) GCD profiles, and (h) long-term cycling performance |
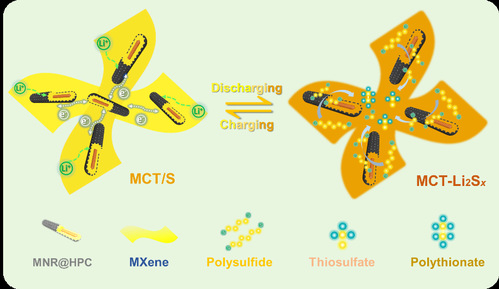 |
| Figure 3 Schematic illustration for the LiPS adsorption/conversion of MCT |