Fabrication of copper foam-lithium composite anode with superior conductivity and robust cycling stability for high-performance anodes in lithium metal batteries.
随着人们对锂离子电池能量密度要求的不断提高,现有的石墨/过渡金属氧化物体系已经很难满足超高比能量的要求,金属锂由于其高的理论比容量和低的电化学电位而被认为是最具前景的负极材料。然而,电池循环过程中锂的不均匀沉积会产生“枝晶”,不断生长的锂枝晶会戳破电池隔膜造成电池内部短路,引起着火甚至爆炸,有极大的安全隐患。同时,“死锂”的产生导致的活性物质的下降和电解液的持续消耗会造成电池容量的衰减和库伦效率的下降,这些问题极大地阻碍了锂金属负极的商业化应用。
Traditional carbon-based/ metal oxide-based anodes cannot meet the rapid growing social demands of electrical energy storage for renewable energy resources. Metallic lithium (Li) is regarded as an ideal anode material due to its high specific theoretical capacity and low electrochemical potential. However, the formation and continuous growth of dendrites due to the uneven deposition of Li may puncture through the separator causing short circuit, even fire in subsequent cycles. Nevertheless, the generation of “dead Li” gives rise to the fading of capacity and Coulombic efficiency, all these drawbacks hinder its practical use in rechargeable Li metal batteries (LMBs).
目前已有研究者利用金属骨架预存锂来稳定电极/电解液界面和抑制枝晶,然而这些复合电极并不能保证金属锂与骨架间的紧密连接,从而增加了内阻。
So far, strategies of pre-storing Li into metal matrix for stabilizing the interface between electrode/electrolyte and suppressing dendrites have been raised. However, increased internal resistance induced by the untight connection between metallic Li and matrix may impede the electron/ion transport.
最近,东南大学的孙正明、陈坚教授课题组(共同通讯作者)通过简单的热熔化-灌输方法制备了一种泡沫铜-金属锂复合电极,为了改善熔融锂与泡沫铜不润湿的现象,利用水热法首先在泡沫铜基底上制备了亲锂层ZnO。该电极具有极低的阻抗和非常优异的导电性,促进了离子/电子输运。同时,泡沫铜作为骨架具有非常好的结构稳定性,内部的网络为锂的沉积提供了空间,极大地缓解了锂沉积过程中的体积膨胀。当其组装成对称电池测试时,表现出极低的过电势;与钛酸锂组成半电池时,表现出优异的倍率性能和循环稳定性。该文章发表在ACS Applied Materials & Interfaces,博士研究生秦立光为该论文的第一作者。
Recently, Sun and Chen group in Southeast University reported a thermal infusion approach to fabricate a CuO-Li composite electrode, a lithiophilic layer of ZnO was prepared by hydrothermal method to modify the wettability between CuO foam and molten Li. The as-fabricated composite anode possesses ultra-low resistance and superior conductivity which impedes the electron/ion transport. Meanwhile, the structural stability as well as the inner space supplying for the Li deposition may reduce volume expansion and suppress the formation of dendrites. The symmetric cells consisted of the composite electrodes show ultra-low overpotential. When paired with Li4Ti5O12 (LTO) electrode, the composite anode exhibits extraordinary electrochemical performance. These finds have been published in the ACS Applied Materials & Interfaces. The Ph.D candidate Liguang Qin is the first author.
全文链接:https://pubs.acs.org/doi/10.1021/acsami.8b07362 (点击跳转到文章页)
核心内容如下:
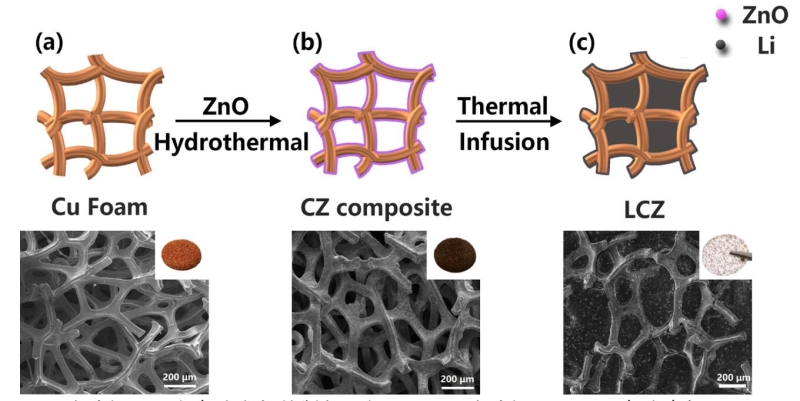
图1. 泡沫铜-金属锂复合负极的制备示意图. (a)泡沫铜,(b)CZ复合电极,(c)LCZ 复合电极
Figure 1. Schematic of the fabrication of the LCZ anode and SEM images of the Cu foam (a), CZ composite (b), LCZ composite (c), the insert images are the optical photographs of the three samples, respectively
首先利用水热法在泡沫铜基底上制备了ZnO亲锂层,然后将包覆有氧化锌纳米颗粒的泡沫铜放入到熔化的金属锂中制备泡沫铜-金属锂复合电极。
Lithiophilic layer of ZnO was prepared by a facile hydrothermal method. Subsequently, the as-prepared Cu-ZnO matrix was transferred into the glove box (H2O < 0.1 ppm, O2 < 0.1 ppm), in which the molten Li infusion was carried out. Firstly, Li foil was scraped with a doctor blade to remove the impurity on the substrate of Li. Then the polished Li foil was placed on an anode shell and then melted at 300°C. As soon as the Li was melted, the as-prepared Cu foam disks were put into contact with molten Li and completed after 30 s, affording an LCZ composite anode.
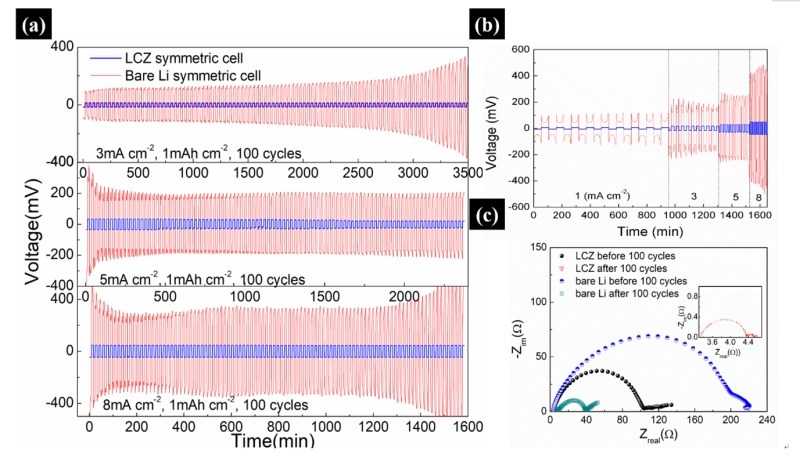
图2. LCZ对称电池和Li对称电池的电化学性能(a)时间-电压曲线;(b)倍率性能;(c)循环前后的阻抗
Figure 2. Electrochemical performance of both symmetric cells. (a) Voltage hysteresis of the LCZ (blue) and bare Li anode (red) symmetrical cell at a current density of 3, 5 and 8 mA cm-2, (b) rate performance, (c) Nyquist plot of the impedance spectra of the LCZ and Li cell before/after 100 cycles at a current density of 3 mA cm-2
当组装成对称电池后,LCZ电极显示出非常优异的电化学性能。当电流密度为3,5和8 mA cm-2时,LCZ电极的过电势分别为15,33和48 mV,且稳定循环100次后没有波动。而未处理锂电极的过电势分别为130,200和400 mV,且曲线波动非常明显。在对电池的阻抗进行测试后发现,LCZ对称电池的阻抗在循环前为100 Ω cm2小于未处理锂电极的220 Ω cm2,循环后LCZ的阻抗为4 Ω cm2左右,远远小于未处理锂电极的40 Ω cm2。如此优异的电化学性能,得益于泡沫铜骨架优良的导电性以及稳定的电极/电解液界面。
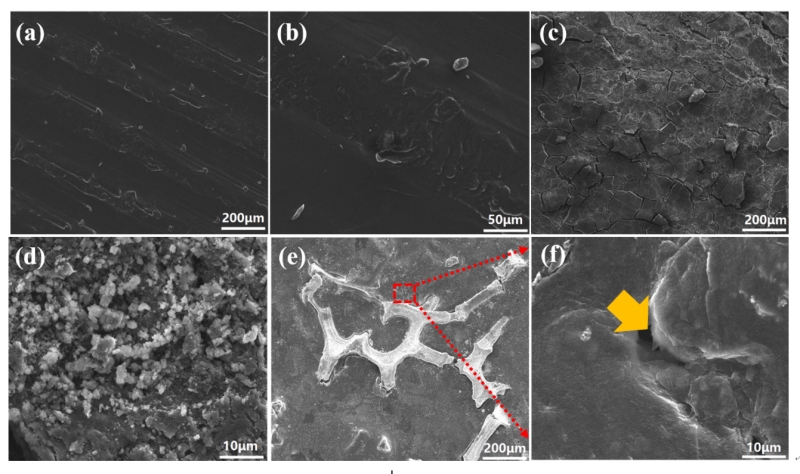
图3. 未处理锂电极循环前的SEM图(a, b);未处理锂电极循环后的SEM图(c, d);LCZ电极循环后的SEM图(e, f)
Figure 3. SEM images of bare Li before (a, b) and after cycling (c, d). SEM images of LCZ after cycling (e, f)
循环前未处理锂电极表面较为光滑,在3 mA cm-2电流密度循环100次后,电极表面出现了很多裂纹且有苔藓状的锂生成。而LCZ电极在循环前后表面形貌基本没有变化,充分说明了LCZ电极的循环稳定性。
As shown in Figure 3 a&b, the bare Li displays a rough surface with uniformly distributed humps before cycling. After 100 cycles at 3 mA cm-2 with a stripping/plating capacity of 1 mAh cm-2, the Li surface becomes rougher (Figure 4c) with the appearance of cracks and mossy Li (Figure 4d). In contrast, the LCZ surface remains almost unchanged after 100 cycles, and only tiny holes appear at the interfaces (Figure 4 e & f). The Cu matrix can still be observed in Figure 4e, the exposed high electrical conductive LCZ surface after Li extraction supplied an electrochemically effective surface area for the Li plating. As a result, the formation of Li dendrites is inhibited and the surface morphology maintains high stability.
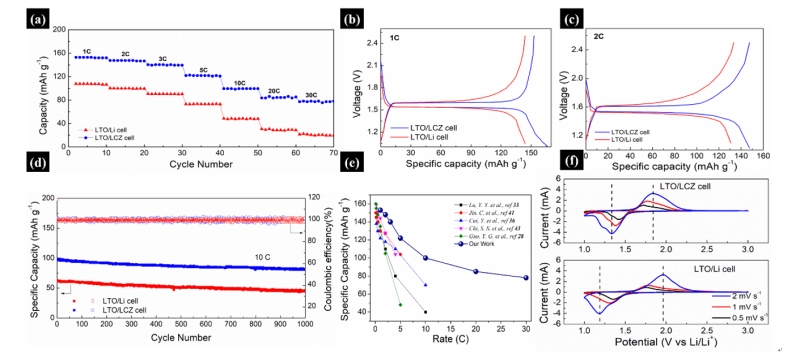
图4. 与LTO组装成半电池后的电化学性能。(a)倍率性能;(b,c)电压曲线;(d)10 C下的长程循环性能;(e)与近期报道的结构设计的锂金属负极的倍率性能对比;(f)LCZ电极的CV曲线
Figure 4. (a) Rate capability of the LCZ composite and the Li anode paired with LTO. (b-c) Voltage profile comparison of the LTO/LCZ cell and the LTO/Li cell at rates of 1C and 2C. (d) Long-term cycling performance of the two cells at 10 C. (e) Comparison of the rate capability of the LCZ anode with the state of the art results in previously reported structure design anode. (f) CV curves of the two cells.
与LTO组装成半电池后,LCZ显示出优异的电化学性能。在10 C(1 C=175 mA g-1)的电流下,1000次循环后容量达到了99 mAh g-1。在1,2,3,5,20,30 C的电流密度下,电池的容量分别为153,148,140,122,85和78 mAh g-1。
When paired with LTO cathode, the LCZ anode exhibited excellent electrochemical properties. It can deliver an ultrahigh capacity of 99 after 1000 cycles at 10 C. Moreover, the capacities of 153, 148, 140, 122, 85 and 78 mAh g-1 were obtained when the rate was 1, 2, 3, 5, 20 and 30 C.
更多学术成果介绍: