用于大体积变化负极材料的一种轮状的蛋黄-壳设计
Toward advanced sodium-ion batteries: a wheel-inspired yolk-shell design for large-volume-change anode materials
蛋黄-壳结构(Yolk-Shell,YS)在缓解锂/钠离子电池合金型负极的巨大体积变化方面展示出巨大的潜力。然而,在传统YS结构中,核和壳之间的点对点接触极大地限制了电子和离子在核壳界面处的传输,而且自支撑的碳壳在长期的循环中可能破损从而导致结构失效。
Yolk-shell structures have found great potential in addressing the huge volume change of alloy-type anodes for lithium-/sodium-ion batteries (LIBs/SIBs).The main challenges associated with yolk-shell structures are the sluggish electron/ion transfer at the yolk-shell interface caused by the point-to-point contact between yolk and shell, and the rupture of self-supporting carbon shells during a long-term cycle.
目前,已有报道通过改变核壳之间的接触方式(例如:线对线和面对面的接触方式)提高YS界面的电子和离子的传输。然而,制备这些精巧的结构通常需要复杂的程序和昂贵的设备,增加了成本。
So far, limited efforts have been made toward the improvement of the electron/ion transfer efficiency by increasing the contact area between yolk and shell (line-to-line and face-to-face models).
最近,东南大学的孙正明、陈坚教授课题组(共同通讯作者)与香港城市大学王钻开教授通过简单可扩展的方法成功合成了一种新颖的轮状蛋黄-壳(Wheel-like Shell, WS)结构。该结构核壳之间通过碳纳米带桥接实现了多点接触,这允许结构内部/外部的电子和离子高效地传输。而且,该结构壳层是由连续的石墨烯网络组成,不仅可以作为导电通路,还可以作为维持结构完整性的机械骨架。当其用于钠离子电池负极时,表现出优异的倍率性能和循环稳定性。该文章发表在国际知名期刊Journal of Materials Chemistry A上(影响因子:8.867),博士研究生徐晖为该论文的第一作者。
Recently, Sun and Chen group in Southeast University with Professor Wang in City University of Hongkong reported a general and facile scaling-up approach to turn a tradition yolk-shell structure (YS) into anovel wheel-shell structure (WS) with multipoint contacts between yolk and shell. The multipoint contact is achieved by bridging SnO2 cores and graphene shells using carbon nanoribbons, which allows a high-efficiency transfer of electron and ion inside/outside the wheel-shell structure. Moreover, the interconnected graphene shells function not only as an electrical highway so that all active materials are electrochemically active, but also as a mechanical backbone to maintain the structural integrity. As an anode for SIBs, the wheel-shell structure exhibits extraordinary electrochemical performance. These findings have been published in the Journal of Materials Chemistry A. The Ph.D candidate Hui Xu is the first author.
全文链接:http://dx.doi.org/10.1039/C8TA03772H
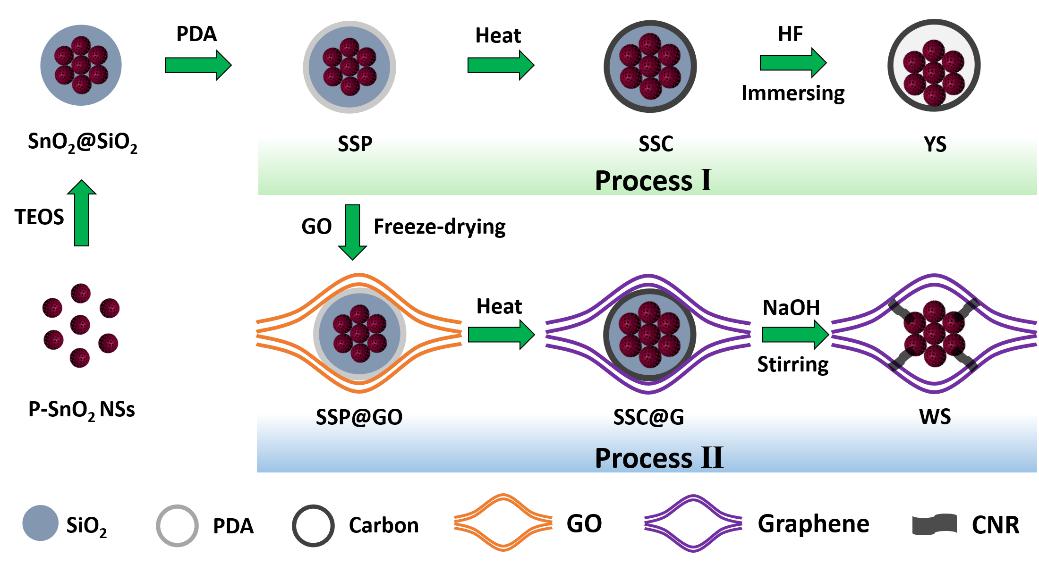
图1.传统的YS结构和新型的WS结构的合成示意图。
Figure 1.Schematic of the fabrication processes for traditional YS structure (process I) and novel WS structure (process II).
首先通过水热法合成了多孔的SnO2纳米球(P-SnO2 NSs),然后依次在其表面包覆SiO2和聚多巴胺(PDA),产物命名为SSP。合成传统的YS结构,只需要将SSP颗粒在氩气中热处理,然后在HF溶液中刻蚀掉SiO2层即可。合成WS结构,我们需要将SSP颗粒均匀地分散到氧化石墨烯(GO)溶液中,冻干后在氩气中热处理,然后在热的NaOH溶液中刻蚀掉SiO2层即可。
Porous SnO2 nanospheres (P-SnO2 NSs) were first synthesized by a facile hydrothermal method. The P-SnO2 NSs were then coated with a SiO2 layer and a polydopamine (PDA) layer, respectively. To obtain the YS structure, the SSP particles were directly heated under Ar and thenimmersed in HF aqueous solution. To obtain the WS structure, the SSP particles were encapsulated into graphene oxide (GO) sheets by a freeze-drying process. The obtained SSP@GO foam monoliths were then heated under Ar to reduce the GO and PDA into graphene and carbon. Finally, after etching SiO2 component with the hot alkaline solution under vigorous stirring, the WS structure is obtained

图2. YS的SEM图(a)和TEM图(b和c);WS的SEM图(d-f)和TEM图(g-i)。
Figure 2.Morphology and detailed structure of YS and WS. (a)SEM image and (b, c) TEM images of YS. The inset of (a) is a magnified SEM image of one yolk-shell particle. (d-f) SEM images and (g-i) TEM images of WS. The CNRs are marked by red arrows in (h and i). The inset of (i) is a HRTEM image of a selected region (a white square in Figure 3i) of WS.
与传统的YS结构相比,WS结构展示出许多优异的结构特征:(1)核壳之间多点接触;(2)三维连续的导电网络;(3)石墨烯和碳纳米带维持结构稳定性;(4)多级孔结构促进电解液渗透。
Fundamentally different from traditional yolk-shell structures, our material design integrates several unique features, such as the multipoint contact between yolk and shell, interconnected 3D conductive network,mechanical stability from the robust graphene shells and CNRs, hierarchical porosity, as well as the admirable synergistic coupling manner between the components.

图3. YS和WS的Raman光谱(a),XRD谱(b)和TGA曲线(c);WS的N2吸附脱附曲线(d)和孔尺寸分布图(d的插图)。
Figure 3. (a) Raman spectra, (b) XRD patterns, (c) TGA curves of WS and YS. (d) Nitrogen adsorption-desorption isotherms of WS. The inset of (d) is the BJH pore size distribution plot.
碳纳米带桥接SnO2核和石墨烯壳是实现WS结构的关键。为此,我们研究了碳纳米带与核壳之间的交互作用。如图4所示,与刻蚀前驱体SSC@G相比,WS的C-N键强度明显下降,表明刻蚀过程导致一部分酰胺键断裂。这引起碳层从石墨烯壳上脱落,并在表面张力的驱使下形成带状结构,即碳纳米带。在完全移出SiO2层之后,碳纳米带可以化学键合在SnO2表面,WS中急剧增强的Sn-C和Sn-O-C键证实了我们的结论。这种化学键合可以提供更强的界面吸附力,不仅能增强电子传导,也能稳定结构。
The bridging between SnO2 cores and graphene shells by CNRs is the critical step that is needed to achieve this novel WS structure. As shown in Figure 4, compared with the precursor (SSC@G), the visible drop of the C−N fraction for the WS suggests that the amide bonds werepartly broken in the hot alkaline solution. This can cause the carbon layer to detach from graphene sheets at the weak bonding areas under vigorous stirring, thereby spontaneously organizing them into a ribbon-like structure (CNR) due to the surface tension. After completely losing the support of the SiO2 layer, the CNRs can be chemically bonded on the surface of the SnO2 yolks, which can be confirmed by therapidly increased Sn−C and Sn−O−C fractions in WS. The chemical bonding may lead to higher interface adhesion strength between them, which is helpful to not only electron transport at these interfaces but also the mechanistic stability.
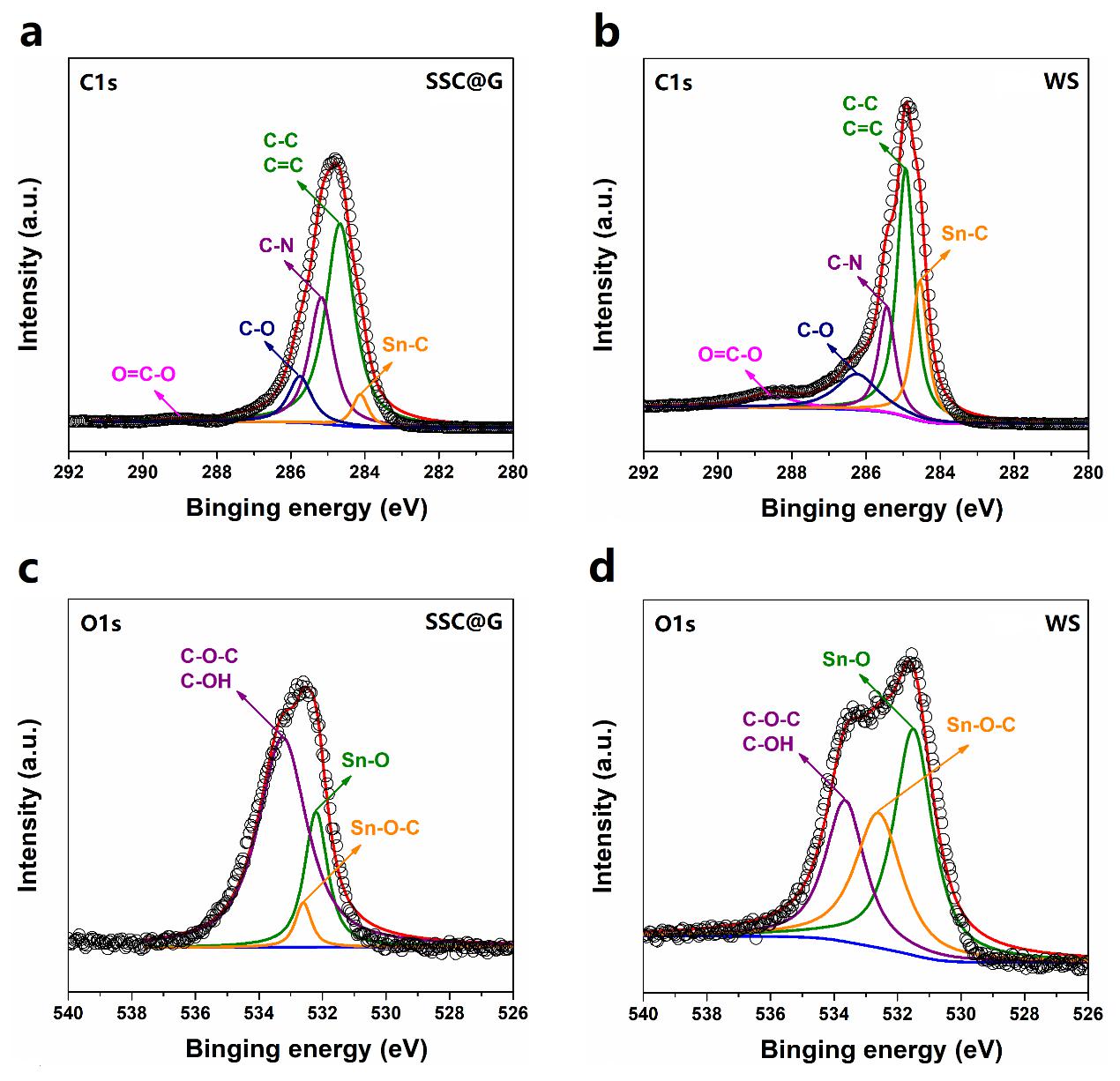
图4.前驱体SSC@G和WS的XPS C1s谱(a和b)和O1s谱(c和d)。
Figure 4. XPS characterization of the interactions between CNRs and graphene shells/SnO2 yolks. (a, b) C1s and (c, d) O1s spectra of SSC@G and WS.
当被用作钠离子电池负极时,WS展示出优异的电化学性能。在1.0A/g的电流密度下,1000次循环后可逆容量高达248.2mAh/g,容量保持率为86.9%(与第一次循环充电容量相比),库仑效率几乎为100%。在0.1, 0.2, 0.5, 1.0, 2.0, 5.0, 10.0A/g的电流密度下可逆容量分别为418.8, 324.3, 266.1, 232.6, 203.2, 177.0和153.3mAh/g;当电流密度恢复到0.1A/g时,容量恢复并保持在353.3mAh/g。
When used as an anode material for SIBs, the WS electrode exhibits excellent electrochemical performance. It can deliver an ultrahigh reversible capacity of 248.2mA h g−1 after 1000 cycles, and the capacity retention ratio reaches up to 86.9 % (based on the first charge capacity). Also, nearly 100% CE is achieved throughout the overall cycling operation. Moreover, the reversible capacities are 418.8, 324.3, 266.1, 232.6, 203.2, 177.0 and 153.3 mA h g−1 at 0.1, 0.2, 0.5, 1.0, 2.0, 5.0 and 10.0 A g−1, respectively. When the current density finally returns to its initial value of 0.1 A g−1, a capacity of 353.3 mA h g−1 is still recoverable, indicating the superior rate capability of the WS electrode.
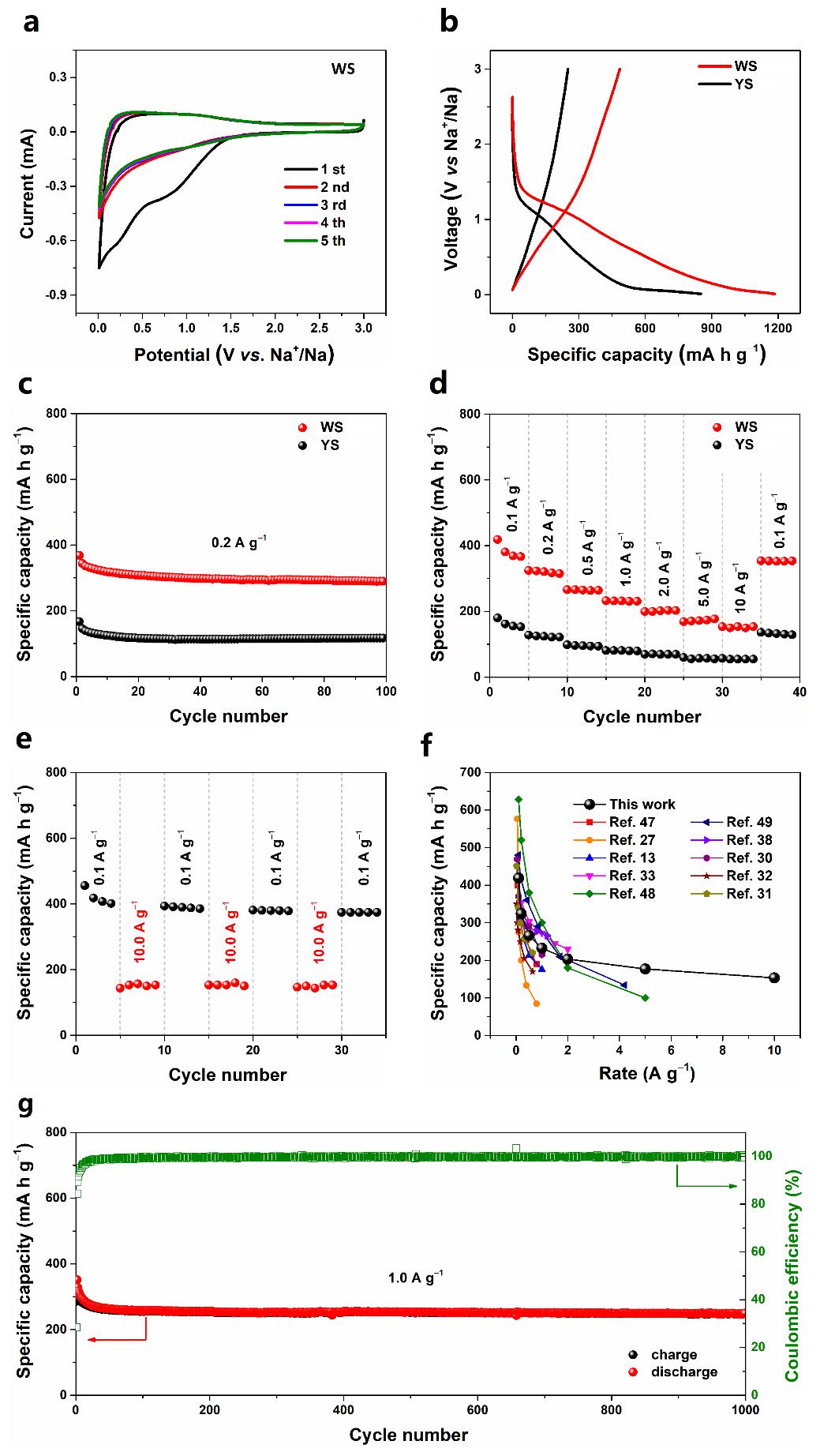
图5. WS的CV曲线(a),WS和YS的首次充放电曲线(b),循环性能(c)和倍率性能(d),WS在交替变化的电流密度下的循环稳定性(e),WS与近期报道的SnO2基钠离子电池负极材料的倍率性能比较(f),WS的长程循环性能(g)。
Figure 5.Electrochemical performance of WS and YS electrodes. (a) Cyclic voltammograms of WS electrode at a scan rate of 1 mV s−1.(b) Initial charge/discharge voltage profiles of WS and YS electrodes at a current density of 0.05 A g−1. (c) Desodiation capacity for the first 100 cycles of WS and YS electrodes at 0.2 A g−1. (d) Rate capability from 0.1 to 10.0 A g−1 for WS and YS electrodes. (e)Cycling stability of WS electrode measured at the alternative current densities of 0.1 A g−1 and 10.0 A g−1. (f) Comparison of the rate capability of WS electrode with the state of the art results in previously reported SnO2-based systems. (g) Long-termcycle performance and corresponding Coulombic efficiency of WS electrode at a current density of 1.0 A g−1.
更多学术成果介绍: